Science
 INSIDE THE NEURON
INSIDE THE NEURON
Let's open up the hood.
by John McManamy

Let's open up the hood.
by John McManamy
HUSSEINI MANJI worked at the NIMH back in its glory days of research during a time roughly coinciding with my entry into mental health writing. This would take us through most of the first decade of the millennium. My introduction came via some research of his I came across showing that lithium – used in treating bipolar – helped make brain cells grow.
Two or three years earlier, Fred Gage of the Salk Institute discovered that, contrary to what we were taught, the brain can actually produce new neurons, a process called neurogenesis. This occurred in the hippocampus, a region of the brain involved in memory and emotions.
At about the same time, Ron Duman at Yale discovered that serotonin-enhancing antidepressants encouraged hippocampal neural growth in lab rats. At first, I thought the fact that psychiatric meds could act as brain fertilizer was the story.
No, Dr Manji told me. Sure, the fact that the brain could grow new cells was important, but the real story, he said, was in how these new and regenerated brain cells connected to other brain cells.
Let’s take it back one step further: Earlier experiments by Robert Sapolsky of Stanford found that animals subjected to stress resulted in dead or atrophied neurons in the hippocampus. The atrophied neurons were more likely to die when subjected to a subsequent stressful event.
But suppose that process could be reversed? Let's return to Dr Duman's research: In his experiments, Dr Duman and his team exposed lab rats to repeated foot shocks to induce behavioral helplessness equating to depression. When the rats were "depressed," neurogenesis virtually shut down. But when the animals were treated with antidepressants, the process was reversed. Neurogenesis cranked up and the little guys were happy again.
Subsequent studies found that both these new cells and restored older cells established connections with existing neural systems. Depleted dendritic spines (responsible for receiving communications from other neurons) grew back. Weakened brain pathways became stronger.
If you are picking up on a common theme on the articles in this section, it’s that our genes are all about how we react to our environment. Vulnerable brains easily get stressed - and overwhelmed - by life events. When our neurons are compromised and our circuits go off-line, all manner of bad things may happen: Depression, anxiety, socially unacceptable behavior. But we are not necessarily victims of our genetics. The brain is plastic. It can grow new cells. It can lay down new neural connections.
It’s a multifaceted topic that can be approached from many angles. My introduction happened to be from inside the neuron. As I heard Dr Manji explain at numerous conferences, neurons communicate with each other through neurotransmitters, but do not actually go inside the nerve cell. Rather, they are merely the keys that unlock what is going on inside the neuron, "where all the action is."
In the bloodstream, in response to stress, glucocorticoids are looking for an easy target to attack – the heart, the immune system, the brain. Inside the brain, this hormone has the effect of upping glutamate, a neurotransmitter involved in exciting the neuron. Too much excitement, though, can overwhelm the poor cell. One of the casualties inside the neuron is brain derived neurotrophic factor (BDNF), a protein that helps mediate neuron survival, inhibit cell death, and modulate synaptic neurotransmitter activity. Dr Duman and his team found that repeated antidepressant treatment "up-regulates" a signal pathway known as the cAMP-CREB cascade, which in turn acts on BDNF.
Meanwhile, experimenting with lithium and Depakote on brain cell tissue, Dr Manji and his colleagues found that these two completely different compounds indirectly affected some of the same cell pathways associated with cell survival and death. One protective protein that utilizes these pathways is Bcl-2, which in one experiment was doubled by lithium and Depakote administration. Subsequent experiments on rats found that lithium mitigated the effects of lab-induced stroke and led to the growth of new neurons in the hippocampus.
For a budding mental health writer, this was heady stuff.
Putting the Pieces Together
The following PowerPoint presentation that Dr Manji delivered at the 2011 9th International Conference on Bipolar Disorder helps guide us through a very confusing thicket. Never mind the complextites. Focus, instead, on the general picture:
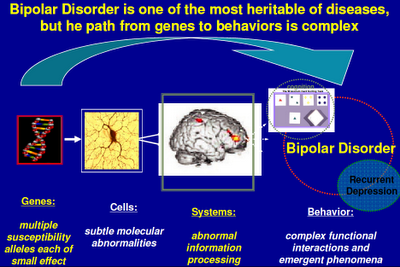
Above: I’ve seen versions of this slide displayed by other scientists from the NIMH. “Alleles” in the first caption refers to genetic variations. Genes switch on proteins that regulate cellular activity. Cells are organized into systems, which in turn influence behavior. (See also in this section Psychiatry's Big Bang.)
SIGN UP FOR MY FREE EMAIL NEWSLETTER
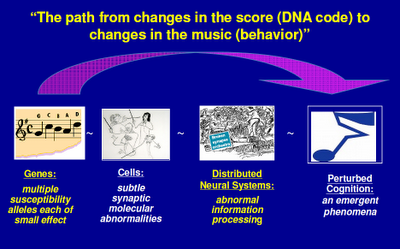
Above: Here’s an overview of what happens when things go wrong. (See also Systems in Collapse.)
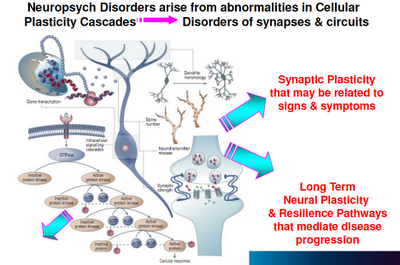
Above: “Plasticity” is the operative word, here. When neurons are compromised in their capacity to maintain cellular function, grow, and connect to new neural networks, bad things happen.
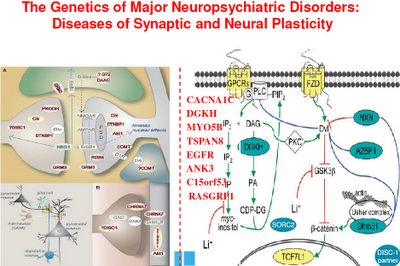
Above: Here are some of the candidate genes that may affect plasticity.
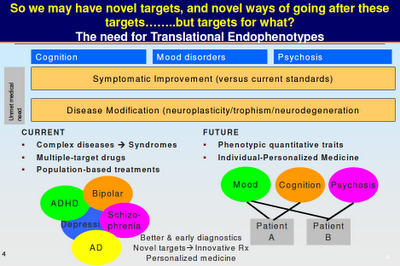
Above: Maybe you can see where Dr Manji is going with this. We are not talking about “bipolar genes” or “schizophrenia genes”. We are talking about genes that affect particular brain functions, which in turn influence how we think and behave. Note the overlap between the various mental illnesses. Note how mood and cognition and psychosis are not restricted to any particular diagnosis. “Phenotype” is the traditional way of looking at mental illness, as symptom clusters. “Endophenotype” looks at what else may be going on (such as a breakdown in neural plasticity).
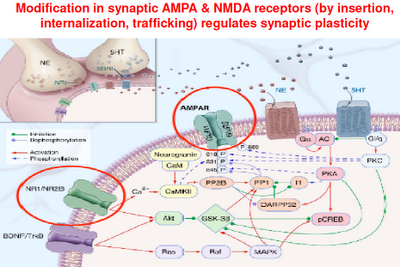
Above: This is a representation of various signaling cascades inside the neuron that regulate neuroplasticity. If the receptors that feed neurotransmission into the cell aren’t functioning right, intracellular signaling is compromised. If intracellular signaling is compromised, the neuron atrophies and may die. This in turn compromises the neuron’s ability to connect with other neurons (through neurotransmission). Whole neuronal networks (synaptic plasticity) are in turn compromised.
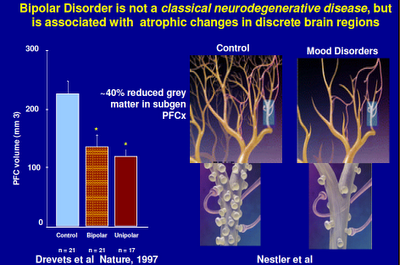
Above: Here we see a representation of a healthy neural network and an unhealthy one. Think of a shriveled tree with few branches.
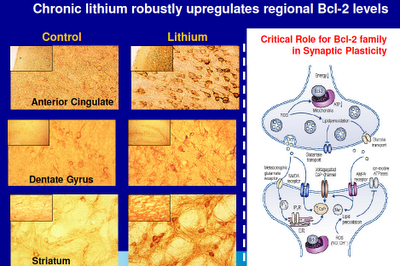
Above: The anterior cingulate, dentate gyrus (part of the hippocampus), and the striatum are all prime suspects when things go wrong with us. The anterior cingulate plays a major role in modulating brain function and in neural connectivity. The hippocampus is where memories are laid down and where new brain cells grow. The striatum is intimately tied to the dopamine system. Note the differences in neural density in these regions with the administration of lithium. Bcl-2 is a protective protein that regulates programmed cell death.

Above: Dr Manji's summary slide. See? Simple, really.
For how the inside-the-neuron "micro" view interacts with a brain systems "macro" view, see Systems in Collapse.
This article has had numerous incarnations since I first published it in 2000. Latest major incarnation 2012, extensively edited Dec 20, 2016.