Science
 DOPAMINE: SEROTONIN'S SECRET WEAPON
DOPAMINE: SEROTONIN'S SECRET WEAPON

THE FOLLOWING is drawn in large part from a review article by Boadie Dunlop and Charles Nemeroff, both then of Emory University, in the March 2007 Archives of General Psychiatry:
The first part of their article focuses exclusively on dopamine’s influence on depression. Yes, serotonin is the neurotransmitter we tend to think of when it comes to depression, but, as the authors point out, a large number of animal studies, plus gene research and neuroimaging on humans and other findings, "support the hypothesis that major depression is associated with a state of reduced dopamine transmission."
The Trouble with Serotonin
The research that led to the "monoamine hypothesis of depression" of the 1960s clearly fingered dopamine, along with serotonin and norepinephrine. ("Chemical imbalance of the brain" is a bastardization of this hypothesis.) But tricyclic antidepressants were hitting the scene at about the same time, all but assuring that dopamine would be overlooked. The later emergence of SSRIs meant that serotonin would grab all the attention. This despite the fact, as the authors point out:
Most antidepressant treatments do not directly enhance dopamine neurotransmission, which may contribute to residual symptoms, including impaired motivation, concentration, and pleasure.
The authors go on to say that "anhedonia, the absolute or relative inability to experience pleasure, is one of two symptoms required for the diagnosis of major depression." Anhedonia is universally regarded as a "core" symptom of depression. It is well-established that dopamine plays a central role in the brain’s reward system, which includes inducing feelings of pleasure and positive mood states.
The authors cite a 2005 University of Toronto study of the brain scans of 24 subjects who were administered amphetamine. The subjects who were severely depressed had a hypersensitive response to the rewarding effects of the drug, and their scans revealed activation in areas of the brain identified in pleasure and reward, including the ventrolateral prefrontal and orbitofrontal cortices, and in two subcortical regions of the brain (the caudate and putamen).
As Dunlop and Nemeroff explain: "These findings further implicate dopamine circuit dysfunction in major depression."
In this context, the first generation of antidepressants may be the most modern. MAOIs prevent degradation of dopamine, as well as norepinephrine and serotonin. For individuals with atypical depression (leaden, fatigued, and lethargic describe some of the characteristics), MAOIs may represent an early treatment option rather than a late one.
SIGN UP FOR MY FREE EMAIL NEWSLETTER
The tricyclics that came out around the same time as MAOIs may also indirectly boost dopamine in the prefrontal cortex via their norepinephrine action.
Enter the new generation antidepressants such as SSRIs. Ironically, as the authors point out, when SSRIs work well it may be due to interactions between the serotonin system and the dopamine system. The authors cite a 1996 German study that found that those who responded to SSRIs - but not those who failed to respond - "exhibited increased dopamine binding to D2 receptors in the striatum and that the degree of increase in D2 binding correlated with improvement in Hamilton Depression Scale score."
In other words, dopamine may be serotonin’s secret weapon.
Dopamine Pathways
If "Dopamine" were the category on "Final Jeopardy," would you be confident in wagering everything?
Most dopamine-producing neurons are located in areas near the brainstem. Their axons extend in one of three specific but overlapping paths (via the medial forebrain bundle) to stimulate specific cortical and subcortical structures. In contrast, serotonin and norepinephrine patterns of distribution are far more diffuse.
The nigrostriatal pathway (in the subcortical areas of the brain) has a prominent role in motor planning and movement, plus cognition. The mesocortical pathway, which projects to the frontal and temporal cortices, is believed to be vital to concentration and executive functions such as working memory. The mesolimbic pathway, which projects into the limbic system, including the hippocampus and amygdala, "is particularly important for motivation, the experience of pleasure, and reward."
The Dopamine Life-Cycle
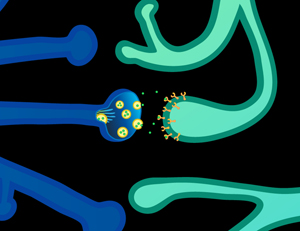
Dopamine is synthesized in the presynaptic neuron from the amino acids phenylalanine and tyrosine. "Phasic" dopamine release is characterized by burst firing and is thought to occur in response to behavioral stimuli, such as those that may predict reward. In contrast, "tonic" dopamine release is slow and irregular.
Dopamine is released from the axon extension of the presynaptic neuron for binding on the postsynaptic neuron at two types of receptors: the dopamine 1 (D1) family, comprising D1 and D5 receptors, and the D2 family that includes D2, D3, and D4.
Depending on which type of receptor dopamine binds to, different and even opposite types of chemical activity take place inside the neuron. For instance, binding on the D1 receptor activates "adenylate cyclase" activity while D2 binding reduces this activity.
Dopamine is cleared from the synapsae via reuptake from the presynaptic neuron or is broken down by the enzymes COMT or MAOI. COMT and its "val/met" variation is the object of considerable attention by geneticists, brain scientists, and the pharmaceutical industry.
Dopamice
Much of what we know about the effects of dopamine comes from studies on rodents. Exposure to chronic mild stress leads to an "animal model of depression" characterized by decreased responsiveness to rewards and reduced sexual and aggressive behaviors. "Learned helplessness," the "forced swim test," and "effort expenditure" effectively brow-beat the little guys into a "what’s the use in trying" state, easily observable by decreased locomotion or lack of effort in going after rewards.
"Depressed" rodents demonstrate altered mesolimbic dopamine function, which have been shown to be reversed by antidepressants (including SSRIs) or prevented by dopamine agonists.
A favorite science project of researchers is engineering "knockout" mice so they lack a particular gene, putting them through the various hoops, and watching what happens. Drs Dunlop and Nemeroff cite a 2005 Harvard study that involved mice lacking a specific "Par-4" function. No, this isn’t about golf, though you could probably get the same result on humans on any given Sunday. Par-4 contains a protein that is involved in programmed cell death and interacts with the D2 receptor from inside the neuron. The Par-4 genes in these mice behaved more like Triple Bogey-7 genes (indicating impaired dopamine signaling inside these cells). As a result, the poor creatures cursed like sailors, broke their five-irons over their knees, and vowed to spend more time with their families, or something like that.
The Dopamine-Serotonin Convergence
SSRIs typically take weeks to achieve their clinical benefit, long after they have supposedly accomplished their chemical mission. Say Dunlop and Nemeroff: "This temporal discrepancy implies that other mechanisms must be involved in recovery from a depressive episode."
The authors refer to "substantial interaction" between the serotonin and dopamine systems in two mid-brain regions (the ventral tegmentum and substantia nigra), with dopamine-producing neurons there being targets for nearby serotonin cells (hailing from the midbrain raphe). In addition, serotonin 1A receptor activation stimulates dopamine release in the prefrontal cortex and in the midbrain (in the nucleus accumbens), at the same time inhibiting dopamine release in the dorsal striatum area of the midbrain. Meanwhile activation of serotonin 2C receptors inhibits mesocortical and mesolimbic function.
Chronic treatment with serotonin antidepressants may moderate the action of serotonin 2B and 2C receptors found on dopamine neurons in the ventral tegmentum area in a way that "may contribute to the amelioration of dopamine-related depressive symptoms."
At the moment, this conversation is mostly "food for thought."
Dopameds
Finally, a brief rundown on dopamine-enhancing antidepressants and dopamine meds being investigated for antidepressant possibilities:
- MAOIs, which prevent degradation of dopamine, serotonin, and norepinephrine, have greater efficacy than tricyclics for atypical depression and "anergic" bipolar depression.
- Wellbutrin blocks dopamine reuptake, but only binds to 22-26 percent of the dopamine transporter sites (by contrast SSRIs block 80 percent or more of serotonin transporter sites).
- Psychostimulants such as Ritalin were found by two studies to be inferior to tricyclics and MAOIs for treating depression.
- Parkinson’s meds act as D2 agonists. Three of them - Parodel (bromocriptine), Travastal (piribedil), and Permax (periglide) – have shown promise for treating depression in small studies.
- Another Parkinson’s med, Mirapex (pramipexole), favors the D3 receptor, and shows promise in small studies for treating unipolar depression and bipolar depression.
In the Meantime, We Are Stuck With Dumb Meds
The April 2007 American Journal of Psychiatry features an editorial by Bruce Cohen and William Carlezon, both of Harvard, titled "Can’t Get Enough of That Dopamine." As the authors point out, "through their many connections, dopamine neurons participate in the modulation of expectation, reward, memory, activity, attention, drives, and mood - the very substrates of psychiatric illness."
Not surprisingly, dopamine dysregulation is implicated in schizophrenia, bipolar, depression, ADD, and substance use. Stimulants and various recreational drugs enhance dopamine signaling while antipsychotics set up a dopamine blockade.
Now here’s the catch. The editorial cites a University of Toronto study appearing in the same issue that shows what dopamine blockade does throughout the brain. Yes, antipsychotics work well in clearing up mania and psychosis, but often at the expense of making patients feel worse.
The study involved 12 patients diagnosed with schizophrenia who had been placed on either Zyprexa or Risperdal. They were given PET scans and queried about their subjective feelings. Those who reported lower states of well-being and higher dysphoria tended to turn up scans that showed greater dopamine blockade.
The striatum region of the brain, above the brainstem, is "dopamine central." From there, dopamine-active neurons project into other brain regions. The study found that blockade of dopamine receptors in the striatum strongly corresponded to a sense of impaired mental function. The study also found that blockade in "extrastriatal" regions such as the temporal lobe correlated to altered emotional regulation.
The effect is about as subtle as the IRS freezing all your bank accounts. True, your mania or psychosis may be gone, but now you may not be able to adequately think or feel. Who wants to live like that? Naturally, the patient gets blamed for noncompliance. According to the editorial:
Without adequate dopamine signaling, our patients do not feel "well." When dopamine systems are dysfunctional, patients seek a change. This may involve stopping a medication, such as antipsychotic drugs that block dopamine. Alternatively, it may be manifest as taking a drug, such as cocaine, that enhances dopamine activity. Either way patients are probably seeking to restore dopamine function.
Obviously, for a lot of us, antipsychotics and stimulants are a godsend. But we also know that our dopamine meds are too dumb to figure out in which regions of the brain they are not wanted. Our current inventory works directly on the dopamine system, but the editorial suggests that a smarter strategy may lie in developing meds with an indirect effect. One possibility is targeting the endorphin, dynorphin, whose neurons are engaged in a feedback loop with dopamine. Another is by learning more about the intracellular signaling connected with dopamine transmission and reception.
In the meantime, we are stuck with the low-tech solutions of considering easing off on antipsychotics doses and just saying no to (street) drugs. Maybe your psychiatrist can come up with some creative work-arounds, but first he or she needs to listen your concerns, really listen.
May 9, 2007, revised Dec 31, 2016
NEW!
Follow me on the road. Check out my New Heart, New Start blog.